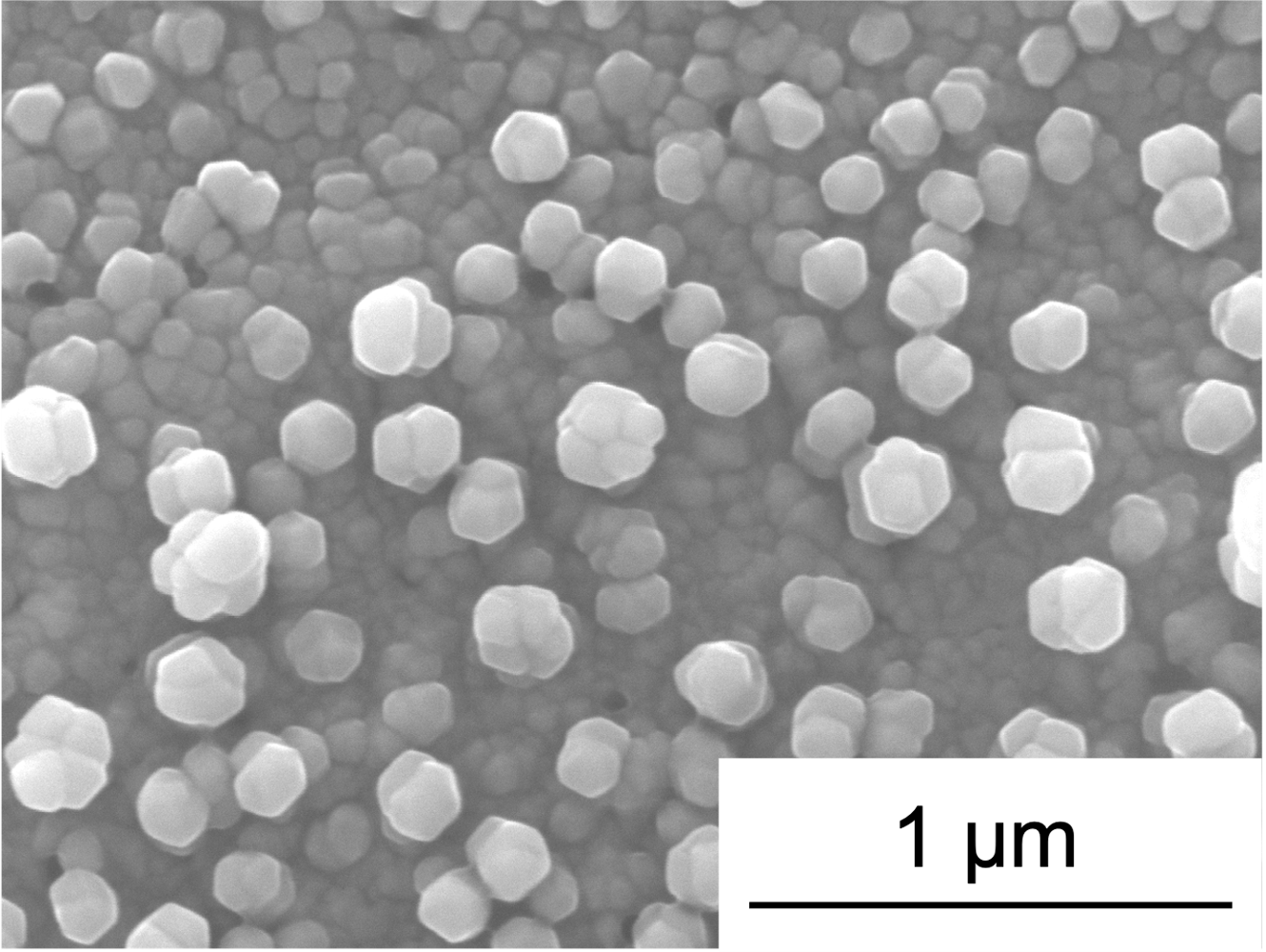
Tin (Sn) and antimony (Sb) are both promising anode materials for sodium-ion batteries, with high theoretical capacities relative to the commercial lithium-ion battery anode (847 and 660 mAh g-1 for Sn and Sb, respectively, compared to 330 mAh g-1 for the standard lithium-ion battery anode, graphite).
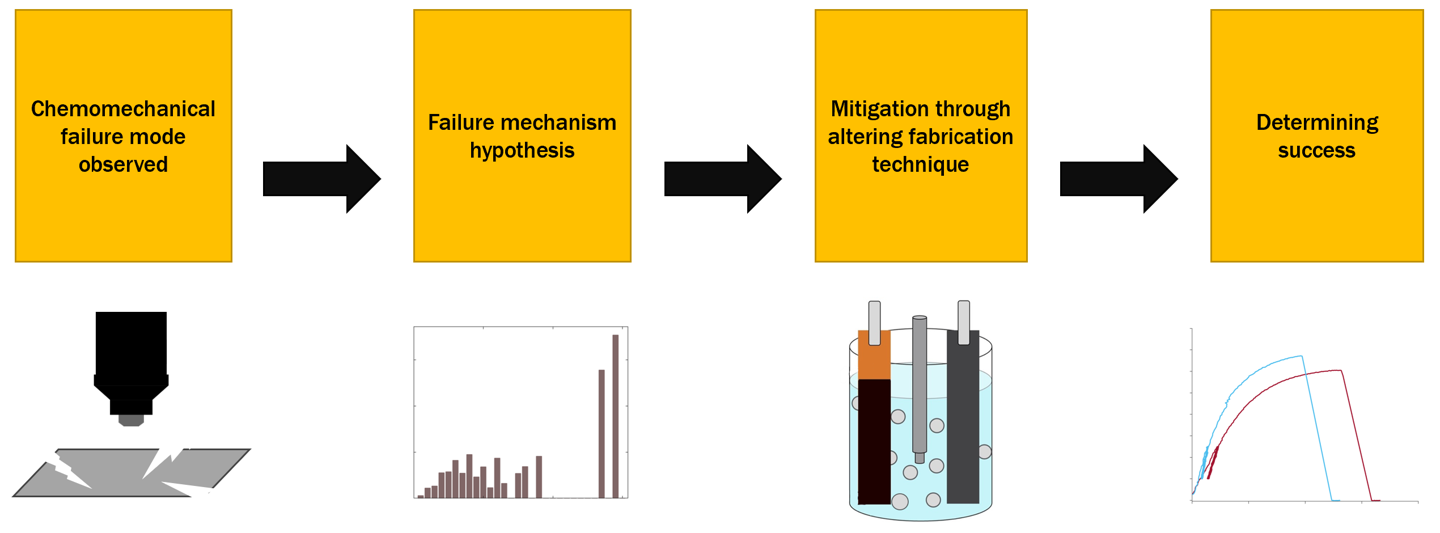
For battery materials, more-traditional experiments require measurements to be taken before cell operation and after cell death and then extrapolating what happened in the interim, creating a "black box" effect. We employ techniques to inform failure modes through measurements taken under battery operating conditions, or in operando. These methods aim to simplify the failure analysis of anodes in batteries towards more informed mitigation strategies and materials design.
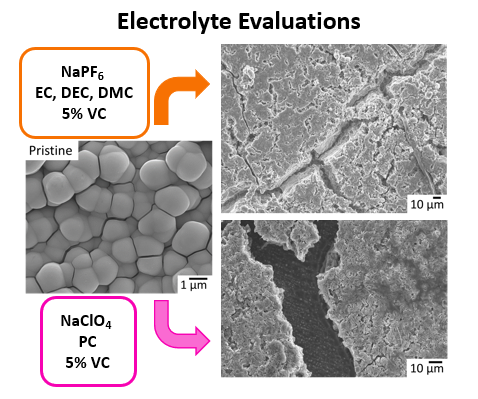
Alkali-ion battery components have an interdependent relationship and in order to commercialize sodium-ion batteries, an understanding of how these components interact with each other is needed. By evaluating the effect of electrolyte composition on electrochemical performance, we can gain insight into optimal electrolyte compositions for anode materials. By formulating unique combinations of salt, solvent and additive, it was found that the electrolyte played a critical in cycle lifetimes and specific capacity achieved.
